Recent News
Business
Manly E. Hogg Unfolds a Tale of Love and Identity in “Born To Be Colored Together: Not Your Ordinary Love Story”
Columbia, SC – WEBWIRE – Thursday, April 25, 2024 In Born to be Colored Together: Not Your Ordinary Love Story, readers are invited into the vibrant world of Dawn Whitecloud, a young woman with big dreams and an even bigger heart. Set against the backdrop of college...
The Book “From Meaningless to Purposeful” by Crystal Summers Will Be Displayed at the Los Angeles Times Festival of Books 2024
Be inspired by a true story about a significant period in the authors life when she experienced tough challenges. In doing so, she developed a loving relationship with Jesus Christ that caused her to live purposefully. San Diego, CA – WEBWIRE – Thursday, April 25,...
“Blackberry Winter: Dormant Vines” by Brenda Heinrich Higgins was displayed at the 2024 L.A. Times Festival of Books
Written as a diary spanning 17741804, Blackberry Winter is firmly rooted in local community life and history. The Deremer familys challenging life is followed from Doylestown, Pennsylvania, to the "untamed frontier" of Mansfield Woodhouse, New Jersey. San Diego, CA –...
Brigit Hits New Growth Milestones as it Distributes More Than $2.3B in Cash Advances and Saves Everyday Americans $1B in Overdraft Fees
NEW YORK, NY – WEBWIRE – Thursday, April 25, 2024 New York City, April 24, 2024 Brigit, the leading financial health app dedicated to improving financial inclusion in America, announces a series of financial milestones, including $100M in revenue and a full year of...
Jeff Martin Auctioneers to Manage Sale of Sabine Mining Company Assets for North American Coal
BROOKLYN, NY, Apr 26, 2024 - (ACN Newswire) - Jeff Martin Auctioneers, in collaboration with North American Coal, will manage the asset liquidation of The Sabine Mining Company, a lignite mine in Hallsville, Texas. This project begins with the sale of the "Yellow Rose...
Cambridge Isotope Laboratories (CIL) Shows Long-Term Commitment to Xenia, Ohio, Facility With New Land Purchase
TEWKSBURY, MA, Apr 25, 2024 - (ACN Newswire) - Cambridge Isotope Laboratories, Inc. (CIL) has acquired an additional 14.8 acres of land at its Cambridge Isotope Separations (CIS) Xenia, Ohio location. Mike Steiger, Vice President of Engineering and Project Execution...
Black Spade Donates Art and Jewellery Collection To Hong Kong Red Cross
HONG KONG, Apr 25, 2024 - (ACN Newswire) - Black Spade Capital Limited (“Black Spade”), the family office of Mr. Lawrence Ho, is delighted to announce its donation of a stunning art and jewellery collection to the Hong Kong Red Cross (“Red Cross"). The gift represents...

Deep Roots Chiropractic Health Center Gets a Website Makeover: Streamlined Experience for Enhanced Patient Care
Bentonville residents seeking natural healthcare solutions now have an easier and faster path to wellness with Deep Roots Chiropractic Health Center’s beautifully redesigned website. The updated website offers a streamlined user experience, improved navigation,...

Shoplooks Makes Digiday Content Marketing Awards 2024 Shortlist in 2 Categories
The Digiday Content Marketing Awards celebrate excellence in modern media and marketing, honoring innovative campaigns, brands, and media organizations. This year's shortlist includes notable names such as Amazon Ads, Reddit, MTV, and now Shoplooks, marking the...



Ammos R Us: Redefining the Frontier of Firearms and Sporting Goods with Its Grand Launch
"Our mission transcends beyond mere transactions; we aim to cultivate a robust community of enthusiasts," proclaimed CSO, Jimmy Slaughter. At Ammos R Us, patrons will discover an elite range of firearms, ammunition, and tactical gear, meticulously curated from...

Watercrest Senior Living Group Welcomes Teleia Farrell to the Market Street Viera Leadership Team
With her background in senior living, Farrell will support the community in sales and operations, ensuring transparency and accountability for family members entrusting their loved one's care and well-being as a resident of Market Street. "Our Market Street Viera...



Southernmost IT, LLC d/b/a GO I.T. Services Announces Relocation of Headquarters to Tampa, Florida
Southernmost IT, LLC, parent company of GO I.T. Services, a leading provider of innovative IT solutions, today announced the relocation of its headquarters from Key West, Florida to the vibrant Tampa Bay area. This strategic move marks a significant milestone in the...

Tampa Surgical Arts Announces a Groundbreaking Collaboration Between World-Renowned Cosmetic Surgeons
Tampa Surgical Arts, a leading, fully accredited surgical facility known for its exceptional patient care and cutting-edge cosmetic surgery techniques, is excited to announce a groundbreaking collaboration that promises to set new standards in cosmetic surgery. Dr....
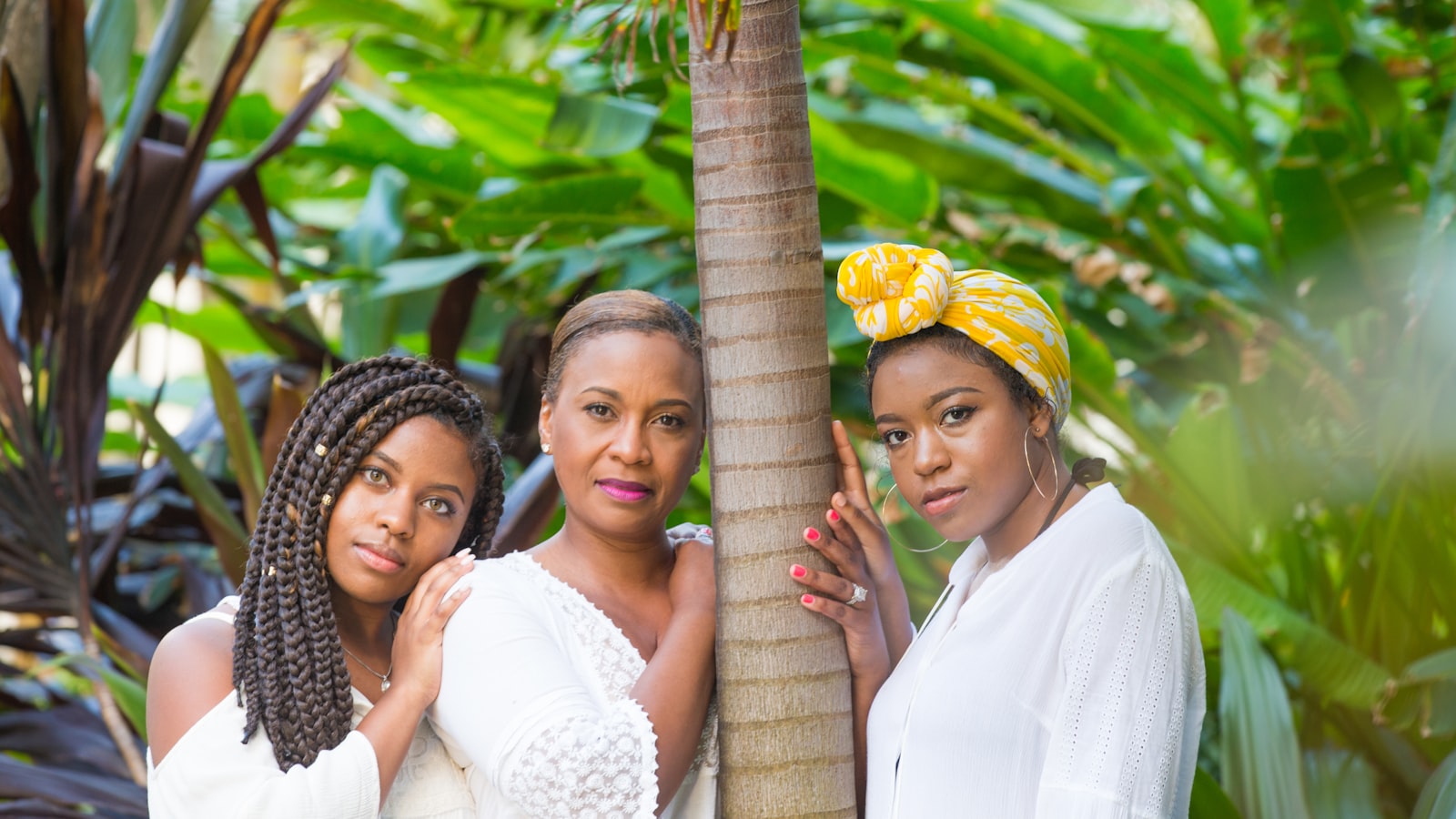
Reel Sisters Celebrates Women in Hip Hop and Honors the Music’s Roots in African Voices Magazine’s Special Issue
On May 17, 2024 at 5:30 pm, Reel Sisters will present an evening of readings and screening of Ladies First: A Story of Women in Hip Hop and Black Love Manifesto. CR Capers, founder of Harlem Film House & the Hip Hop Film Festival, will lead a discussion with Crystal...
Chef Mick Élysée Partners with Enish Dubai for Dubai Restaurant Week
Renowned sustainable Chef Mick Élysée, Joins Forces with Enish, the Largest Nigerian Restaurant Chain for an Exclusive Kitchen Takeover during Dubai Restaurant Week Dubai, United Arab Emirates Apr 24, 2024 (Issuewire.com) - Renowned sustainable Chef Mick Élysée is...
Entertainment
Believe and Jjust Music Join Forces to Revolutionize Bollywood OST Market
Believe, one of the world’s leading digital music companies, and Jjust Music, an acclaimed new-age music label celebrated for its string of Indie music hits, have proudly announced a momentous partnership that will redefine the landscape of the Bollywood Original...
OKIE To Launch 32-inch Sports Series Smart TVs in South India as Demand Surges Amidst Upcoming 2023 Sports Season
Immersive Viewing Experience with 32”, 40”, and 43” Screens Full HD display and 4K Ultra HD resolution for crystal-clear images and vivid colours In-built voice control for seamless navigation 20-watt powerful sound output and built-in soundbars for immersive audio...
Jjust Music launches its first film song Jalsa 2.0, starring Akshay Kumar and Parineeti Chopra
Jjust Music, founded by the Bollywood Luminary and Producer, Jackky Bhagnani, is poised to set the stage ablaze with its inaugural original soundtrack (OST) for the much-anticipated cinematic masterpiece, "Mission Raniganj- The Great Bharat Rescue starring Akshay...
The ultimate celebration of India’s rock music scene: Mahindra Independence Rock unveils its line-up of 10 diverse rock acts for its 29th edition
Following last year’s triumphant return, where Mahindra Independence Rock (I-Rock) reclaimed its rich legacy, India’s legendary rock festival is gearing up to ignite the stage (and mosh pits) once more at Mumbai’s Bayview Lawns this year. This iconic festival’s...
Mark your calendar for the intensive 2-day tattoo workshop and take your skills to the next level!
Join us for an intensive 2-day tattoo workshop and take your skills to the next level! Explore business growth hacks, advanced tattooing techniques, and digital art concepts to enhance your craft. Hosted at Malad, this workshop will provide a unique opportunity to...
Amazon miniTV announces ‘Builders – An Inside story of a modern-day gym!
Amazon miniTV - Amazon’s free video streaming service has been a part of the hit parade with its path-breaking and diverse content library. Adding yet another feather to its hat, the streaming service today announced its latest show, Builders - a unique one-of-a-kind...
Gulf Coast Records’ Blues Music Award-Winner Mike Zito Set to Record New Album Sessions for Upcoming Life Is Hard CD Scheduled for February, 2024
Gulf Coast Records announces a February, 2024, release for the upcoming disc from multi-Blues Music Award-winner Mike Zito, to be titled Life Is Hard. Zito will record the new album September 18-22 during sessions at Sunset Sound Studios in Hollywood, California, with...
Noise announces introduction of Noise Junior, a dedicated smartwatch category for kids in India; launches Noise Explorer
Noise, India's No.1 smartwatch brand, has debuted Noise Junior, the brand's dedicated smartwatch category for kids. Being one of the first to identify the need for smart wearables for kids, Noise today unveiled this new category is tailor-made keeping the children and...
The ultimate celebration of India’s rock music scene: Mahindra Independence Rock unveils its line-up of 10 diverse rock acts for its 29th edition
Following last year's triumphant return, where Mahindra Independence Rock (I-Rock) reclaimed its rich legacy, India's legendary rock festival is gearing up to ignite the stage (and mosh pits) once more at Mumbai's Bayview Lawns this year. This iconic festival's...
DBZ Figure Son Goku Black Hair Version Gift
"DBZ Figure Son Goku Black Hair Version Gift" Celebrates the Legacy of Dragon Ball Z with a Unique Collector's Item Fans of the legendary Dragon Ball Z series have a reason to rejoice as [Your Company Name], a leading name in collectibles, unveils an exquisite new...
Sports
Chotrani, Anahat emerge champions
Maharashtra’s Veer Chotrani and Delhi’s Anahat Singh maintained their imperious form in the tournament, right till the end, lifting the men’s and women’s singles titles in the 6th Cello NSCI Open National Circuit squash tournament, hosted by NSCI at their glass backed...
magicpin Delivers on Its Promise: Office Signage Transformed into ‘Mahipin’ as MS Dhoni and CSK Triumph in IPL Final
Brace yourselves for the epic transformation that just took place at magicpin - India's first and largest hyperlocal startup headquarters in IFFCO Chowk at Gurugram. In a bold move, magicpin’s office signage has officially become 'Mahipin' to honor the incredible...
Chotrani ousts top seed Baitha to make men’s final
Maharashtra’s Veer Chotrani stormed into the men’s final with a dominating upset of top seed Rahul Baitha in the 6th Cello NSCI Open National Circuit squash tournament, hosted by NSCI at their glass backed squash courts, here, on Tuesday. The tournament is...
Sakib gets better of Chauhan to enters semis
Jamal Sakib of Services, seeded 5/8, scored a minor upset over his Services opponent Vaibhav Chauhan (3/4) to enter the men’s semi-finals of the 6th Cello NSCI Open National Circuit squash tournament, hosted by NSCI at their glass backed squash courts, here, on...
Singhva knock out top seed Achpal
Unseeded Naresh Singhva of Maharashtra pulled off the biggest win of his career when he knocked out top seed Adith Achpal of TN in the boys Under-19 pre-quarter-finals of the 6th Cello NSCI Open National Circuit squash tournament, hosted by NSCI at their glass backed...
JioCinema Equals the World Record for Concurrency Clocking 2.5 Cr Viewers During the Qualifier 1 between Gujarat Titans and Chennai Super Kings
JioCinema continued to set new benchmarks as it clocked 2.5 Cr. concurrent viewers during the Qualifier 1 of the TATA IPL 2023 match between Gujarat Titans and Chennai Super Kings. JioCinema not only broke its own record for the third time this season but also...
adidas is unveiling Move For The Planet
Today, adidas is unveiling Move For The Planet; a new global initiative that will harness the collective activity of sporting communities across the world. adidas is encouraging people to turn activity into action as it pledges to donate €1 to Common Goal for every 10...
adidas and Arsenal reveal the Arsenal home jersey
Today, adidas and Arsenal reveal the Arsenal home jersey for the 2023/24 season, marking the 20th anniversary of the ‘Invincibles’ season with a bold new look. Taking design inspiration from the kit worn by the record-breaking team, the jersey features a new shade of...
Reebok launches ‘I am the New’ brand campaign, ropes in leading actor Taapsee Pannu and World’s No. 1 T20 batter Suryakumar Yadav as brand ambassadors
Reebok, a brand synonymous with sports and fitness, has announced a new chapter in its journey. Under the aegis of Aditya Birla Fashion and Retail Limited (ABFRL) in India, Reebok is re-establishing itself as a leading sports and performance brand with a powerful new...
TATA IPL 2023 on JioCinema Clocks a Record-Breaking 1300 Cr. + Video Views in the First Five Weeks
JioCinema, the Official Digital Streaming Partner of TATA IPL 2023, continues to set global benchmarks in the world of digital sports viewing as it clocked over 1300 Cr. video views in the first five weeks. Viewers were glued to JioCinema’s fan-centric presentation as...
Philips Avent encourages mothers to comeback their way with Sania Mirza; launches #AskAvent – a knowledge hub for breastfeeding mothers
Motherhood is one of the most sacred and revered roles that a woman can take on and the journey is even more incredible. However, it comes with its unique set of challenges that can often make it difficult for mothers to pursue their passions and careers. After having...
Pradhan, Gawate win Federal Bank Kochi Marathon 2023
Uttarakhand's Arjun Pradhan and Maharashtra long-distance runner Jyoti Gawate won the inaugural Federal Bank Kochi Marathon 2023, which was organized by CleoSportz, and held here on Monday. Arjun Pradhan, who was the favourite in the men's elite category, emerged...
This 19 years old Three-Star Wide Receiver Deserves Your Attention
There are always players that don’t end up getting the attention they rightfully deserve in both college and professional sports. Here we are hoping to change that regarding one player in particular, DeVante Perkins. Perkins is a name that, if you aren’t already...
National Lacrosse League Announces Information For 2022 Entry Draft
The National Lacrosse League (@NLL), the largest and most successful professional lacrosse property in the world, today announced that the 2022 NLL Entry Draft will be held on Saturday, September 10 at 2 p.m. ET at the historic The Carlu in downtown Toronto....
Joe Moore Award Becomes First Post-Season College Award Platform To Launch NIL Program
The Foundation for Teamwork, owners and creators of the Joe Moore Award (@joemooreaward) which honors the most Outstanding Offensive Line Unit in College Football, today announced that it is believed to be the first post season college award program to launch a Name...
Interviews & Features
Blackjack Review 2022 For Online Gambling- Gambling Sites Club
You’ve probably heard that learning blackjack takes a minute and mastering it takes a lifetime. It’s only fair, because blackjack online is a fantastic game! Right? Let’s take a closer look at this contentious game. What is Online Blackjack Gambling? Blackjack is a...
3 Reasons Why It is a Must to Hire an eCommerce Design Agency
While Considering the website composition and manner in which a web-based store looks, typically the creative viewpoints ring a bell. How a specific picture looks or the varieties that are being utilized. These are absolutely significant components, however there are...
Year Ender Quotes | Women Entrepreneurs
Malini Agarwal- Founder & Creative Director MissMalini Entertainment & Girl Tribe From the onset of the pandemic there has been a spotlight on influencer marketing as a crucial part of the marketing mix. The rapid growth of the creator ecosystem over the last...
‘Puressentiel Purifying Air Spray’ an essential requirement for your home
Pollution has been a concern for us Indians more so over the past few years with the AQI rising above hazardous. It is well known that outdoor polluted air can cause irreversible damage to the respiratory system. However Indoor air pollution is 100 times more...
Recipe – CORNITOS AVOCADO MINI WAFFLES WITH DRAGON FRUIT AND AVOCADO SALSA
Avocado Mini Waffles: Ingredients: For the avocado filling: 1/2 mashed avocado 1/4 cup grated paneer 1 tbsp chopped onion 2 chopped garlic cloves 1/2 chopped green chilli 1/2 tsp mixed herbs 1 tbsp chopped coriander 1/2 tsp lemon juice Salt to taste For waffles:...
Card91 appoints Srijit Sanyal as Vice President, Sales
Card91, a payment infrastructure platform, has appointed Srijit Sanyal as Vice President, Sales & Partnership. A veteran in the Fintech industry, Srijit has nearly 2 decades of rich sales experience with banks, fintechs and pharmaceuticals and has held senior...
All News
Manly E. Hogg Unfolds a Tale of Love and Identity in “Born To Be Colored Together: Not Your Ordinary Love Story”
Columbia, SC – WEBWIRE – Thursday, April 25, 2024 In Born to be Colored Together: Not Your...
The Book “From Meaningless to Purposeful” by Crystal Summers Will Be Displayed at the Los Angeles Times Festival of Books 2024
Be inspired by a true story about a significant period in the authors life when she experienced...
“Blackberry Winter: Dormant Vines” by Brenda Heinrich Higgins was displayed at the 2024 L.A. Times Festival of Books
Written as a diary spanning 17741804, Blackberry Winter is firmly rooted in local community life...
Brigit Hits New Growth Milestones as it Distributes More Than $2.3B in Cash Advances and Saves Everyday Americans $1B in Overdraft Fees
NEW YORK, NY – WEBWIRE – Thursday, April 25, 2024 New York City, April 24, 2024 Brigit, the...
Jeff Martin Auctioneers to Manage Sale of Sabine Mining Company Assets for North American Coal
BROOKLYN, NY, Apr 26, 2024 - (ACN Newswire) - Jeff Martin Auctioneers, in collaboration with North...
Cambridge Isotope Laboratories (CIL) Shows Long-Term Commitment to Xenia, Ohio, Facility With New Land Purchase
TEWKSBURY, MA, Apr 25, 2024 - (ACN Newswire) - Cambridge Isotope Laboratories, Inc. (CIL) has...
Black Spade Donates Art and Jewellery Collection To Hong Kong Red Cross
HONG KONG, Apr 25, 2024 - (ACN Newswire) - Black Spade Capital Limited (“Black Spade”), the family...

Deep Roots Chiropractic Health Center Gets a Website Makeover: Streamlined Experience for Enhanced Patient Care
Bentonville residents seeking natural healthcare solutions now have an easier and faster path to...
Ankura Hospital Vijayawada organised ‘Mommy’s Day Out’
Ankura Hospital for Women and Children organised “Mommy’s Day Out” a day with fun-filled...
Unlocking the Role of Nutritional Snacks in Boosting Immunity and Family Well-being
In the midst of life's hustle and bustle, our busy schedules often lead us to unhealthy snacks,...



Shoplooks Makes Digiday Content Marketing Awards 2024 Shortlist in 2 Categories
The Digiday Content Marketing Awards celebrate excellence in modern media and marketing, honoring...



Ammos R Us: Redefining the Frontier of Firearms and Sporting Goods with Its Grand Launch
"Our mission transcends beyond mere transactions; we aim to cultivate a robust community of...

Watercrest Senior Living Group Welcomes Teleia Farrell to the Market Street Viera Leadership Team
With her background in senior living, Farrell will support the community in sales and operations,...
Narayana Health unveils InsidER – a riveting Medical docudrama series directed by prominent pictures
– In an exciting new venture, Narayana Health has entrusted Prominent Pictures with the creative...
Adani Gangavaram Port initiates special education programs for Gangavaram and Dibbapalem village students
Adani Gangavaram Port is association with Adani Foundation has initiated various sustainable...
College of Physicians and Surgeons of Mumbai (CPS) Addresses Concerns Regarding its Courses and Recognitions
The College of Physicians and Surgeons of Mumbai (CPS) addresses recent misinterpretations and...



Southernmost IT, LLC d/b/a GO I.T. Services Announces Relocation of Headquarters to Tampa, Florida
Southernmost IT, LLC, parent company of GO I.T. Services, a leading provider of innovative IT...

Tampa Surgical Arts Announces a Groundbreaking Collaboration Between World-Renowned Cosmetic Surgeons
Tampa Surgical Arts, a leading, fully accredited surgical facility known for its exceptional...
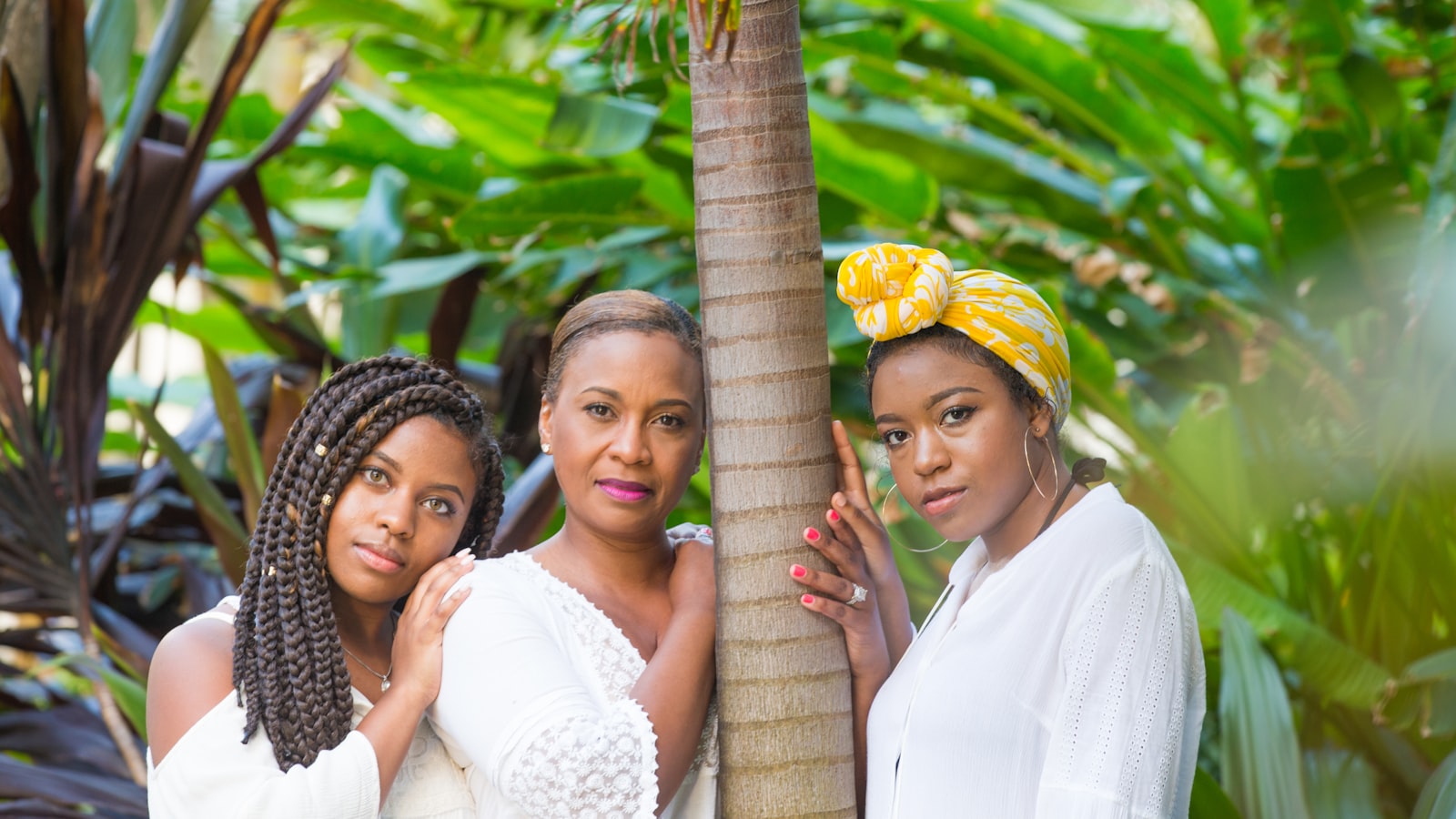
Reel Sisters Celebrates Women in Hip Hop and Honors the Music’s Roots in African Voices Magazine’s Special Issue
On May 17, 2024 at 5:30 pm, Reel Sisters will present an evening of readings and screening of...
Emeritus Claims Top Spot in TIME Magazine’s ‘World’s Top EdTech Companies of 2024’ Ranking
Emeritus, a global leader in delivering accessible and affordable high-quality education, has been...
Arkreach Unveils Revolutionary AI-Powered Contextual Sentiment Analysis, Transforming Communications Analytics Globally
Arkreach, a leader in communications analytics, today announced the launch of its groundbreaking...
Chef Mick Élysée Partners with Enish Dubai for Dubai Restaurant Week
Renowned sustainable Chef Mick Élysée, Joins Forces with Enish, the Largest Nigerian Restaurant...
Get 10% off Any Music Promotion from Music Promotion Club
Music Promotion Club is coming up with a 10% discount for every artist. The offer on Music...
Japan – Mitsubishi Power Begins Commercial Operation of Seventh M701JAC Gas Turbine in Thailand GTCC Project; Achieves 75,000 AOH To-Date
Mitsubishi Power, a power solutions brand of Mitsubishi Heavy Industries, Ltd. (MHI), has...
Japan – Mitsubishi Motors Posts Record Sales in the Philippines in FY2023
Mitsubishi Motors Corporation (hereafter, Mitsubishi Motors) announced that it posted record...
Contribute to Web Newswire
Access Premium Content
You can access and use the content for free on your website if you give an attribution and linkback to us.
Thanks for supporting us!
Contribute your Content
PR Agencies, Brands and others can contribute your content with us for free. This will now be subject to editorial approval. (5pm-6pm IST, All Days in a Week)
Follow Us
Please link us using RSS. We have stopped updating social medias channels for updates so that we can focus on quality content that is more useful for all of us.
