Recent News
Business
Crown Digital Spearheads the Future of AI in F&B with Ella the Robobarista at AIM Global
Crown Digital, Singapore’s first full-stacked F&B tech startup and the creator of Ella the Robot Barista, has joined AIM Global, the Global Alliance on Artificial Intelligence for Industry and Manufacturing. Crown Digital joins AIM Global as the first Singaporean...
Microex Launches Web3.0 Financial Trading Solution – Pioneering Innovation in Financial Technology
NEW YORK, Apr 17, 2024 - (ACN Newswire) - Microex, a UAE-based financial technology company, today announced the launch of its web3.0 financial trading solution. This innovative platform marks a significant step forward in the financial technology industry. Since its...
The Executive Centre Announces Record Revenue in FY2023 Annual Results
The Executive Centre (TEC), the leading premium workspace provider that serves more than 47,000 Members in 34 cities across Asia-Pacific and the Middle East, announced its annual results today for fiscal year 2023. Achieving a record-breaking revenue of USD 315...
All-New Triton Confirmed as First Double-Cab Pickup Truck to Achieve 2024 Five-Star ANCAP Safety Rating
Mitsubishi Motors Corporation (hereafter, Mitsubishi Motors) announced that the all-new Triton(1) one-ton pickup truck has earned a maximum five-star rating(2) in the 2024 ANCAP(3), which evaluates the overall safety performance of new vehicles in Australia and New...

Kalimat Telecom Selects Parallel Wireless for Network Build Out
New Delhi, Delhi, India Parallel Wireless, Inc., the leading provider of the world’s first end-to-end software-based unified network solution across All Gs (2G, 3G, 4G, and 5G), today announced that Kalimat Telecom, a subsidiary of Kuwait’s Trade Links, has selected...

Parallel Wireless’s All-G Technology Ready to Transform Telecom Services in India
New Delhi, Delhi, India Parallel Wireless, Inc., the leader in providing the world’s first end-to-end software-based network solutions for 2G, 3G, 4G and 5G, after globally proving the immense value of its All-G software-enabled solutions is now looking forward to...



Quickparts Achieves Global ISO 27001:2022 Certification, Highlighting Commitment to Information Security
The ISO 27001:2022 certification signifies that Quickparts has implemented a robust Information Security Management System (ISMS) that meets the highest international standards. This system encompasses all aspects of information security, including risk management,...



Unveiling Sapphire, The First True Native Edge Ai Camera Solution, By Kami Vision: A Glimpse Into The Future Of Security
Launching SAPPHIRE! Delve into the cutting-edge of intelligent business technology at ISC WEST from April 10-12, 2024. Experience the future firsthand at Booth #33081 in Las Vegas, NV. Unlike complex systems of the past, SAPPHIRE offers a hassle-free experience with...



R-mb-rger Family Association Lauds Grit in Facing Challenges at 2024 Reunion
Local historian and author Steve Troutman and Family Historian Bob Averell will be joined by guest speaker Ben Ancheff, former pitcher, MLB replay ops administrator, and current athletic director of Williams Valley Jr./Sr. High School. No cover; contributions and...



Philadelphia Phillies and NEST launch Skilled Trades All-Star Program to Ignite Interest in Skilled Trades Among Youth
Local Students to Gain Hands-On Experience at Citizens Bank Park on April 24 and August 1 The Philadelphia Phillies and NEST, the pioneer of integrated facilities management, are joining forces to launch the Skilled Trades All-Star Program, a dynamic initiative aimed...



Dr. Phyllis Worthy Dawkins, HBCU Executive Leadership Institute Executive Director, To Receive the Johnson C. Smith 2024 ‘Arch of Triumph’ Award
Clark Atlanta University’s HBCU Executive Leadership Institute (HBCU ELI) proudly announced that Executive Director and 18th President of Bennett College, Dr. Phyllis Worthy Dawkins, was selected to receive the Johnson C. Smith University (JCSU) 2024 ‘Arch of Triumph...



Melville Law Represents Syndicate of Venture Capital Funds in $6M Seed Investment for Brain Jar Games
$6 million investment from venture capital funds syndicate targeted on boosting the innovative gaming startup’s growth. Melville Law, Melville Law, a legal firm specializing in technology and entertainment, announced its role in representing a syndicate of venture...



One Way Exteriors Announces Relocation to a New Office in Wilkesboro, NC
One Way Exteriors, a leading seamless gutter company serving the North Carolina area, is excited to announce its recent relocation to a new office space in Wilkesboro. One Way Exteriors, a leading seamless gutter company serving the North Carolina area, is excited to...



Bemis Assist Premium Toilet Seat Eliminates Need for Wall-Mounted Rails
Designed for safety and independence in the bathroom, Assist features secure, built-in support arms and a residential appearance Bemis Manufacturing Company, a leading bidet and toilet seat manufacturer, announced the Assist premium standard height toilet seat that...
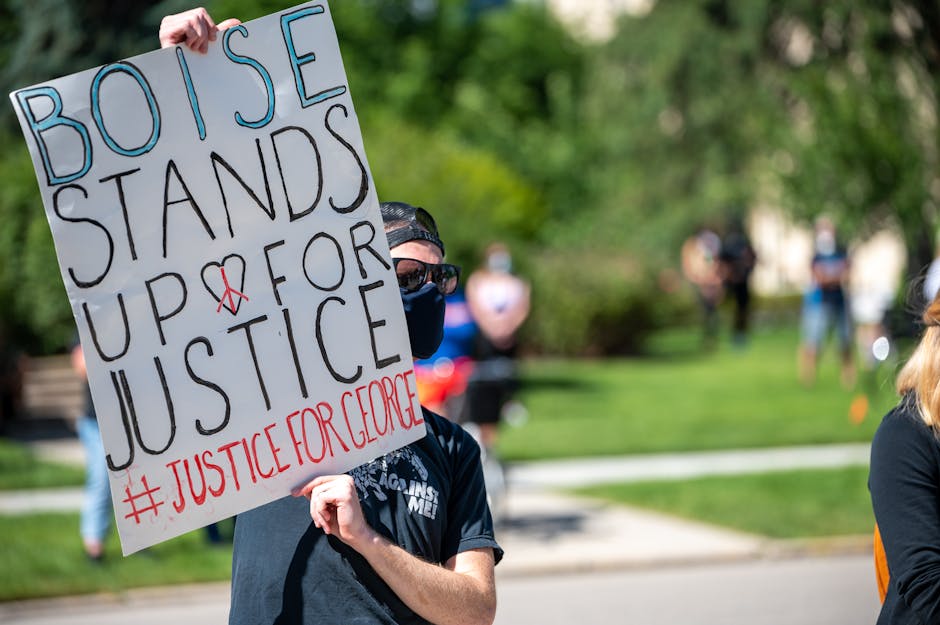
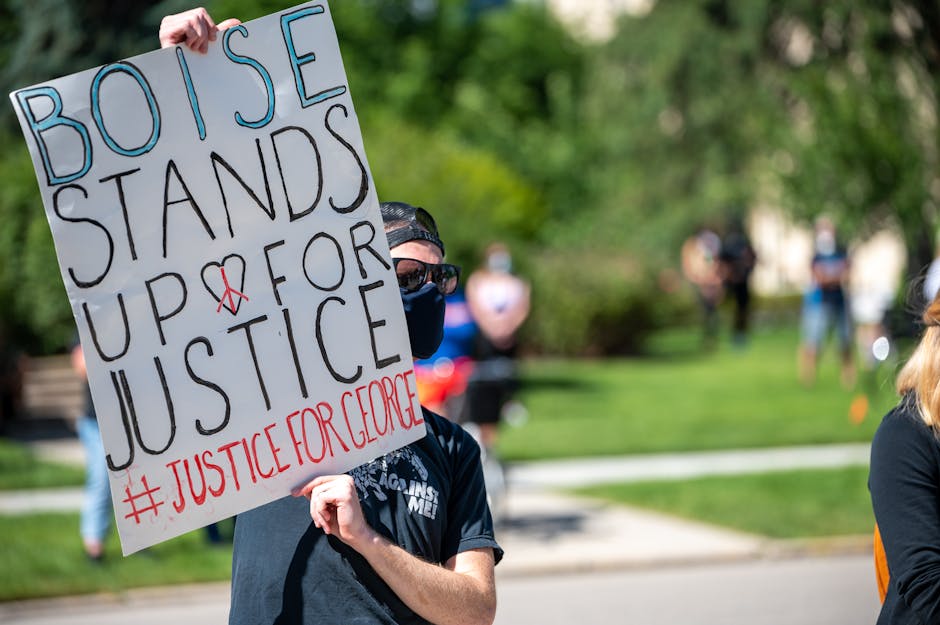
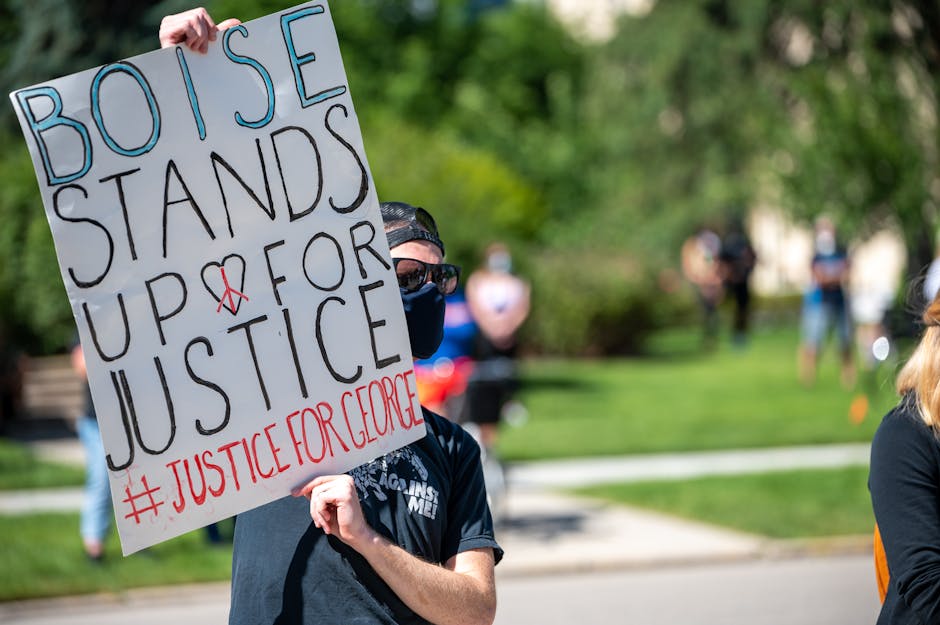
L-Tron to Attend and Sponsor SC IAI Investigators Conference
FORT MILL, S.C. - April 16, 2024 - PRLog -- For the second consecutive year, L-Tron will attend the South Carolina Division of the International Association for Identification (SC IAI) Spring Conference from May 6-9, 2024.This year's conference will be held at Rock...
Entertainment
Believe and Jjust Music Join Forces to Revolutionize Bollywood OST Market
Believe, one of the world’s leading digital music companies, and Jjust Music, an acclaimed new-age music label celebrated for its string of Indie music hits, have proudly announced a momentous partnership that will redefine the landscape of the Bollywood Original...
OKIE To Launch 32-inch Sports Series Smart TVs in South India as Demand Surges Amidst Upcoming 2023 Sports Season
Immersive Viewing Experience with 32”, 40”, and 43” Screens Full HD display and 4K Ultra HD resolution for crystal-clear images and vivid colours In-built voice control for seamless navigation 20-watt powerful sound output and built-in soundbars for immersive audio...
Jjust Music launches its first film song Jalsa 2.0, starring Akshay Kumar and Parineeti Chopra
Jjust Music, founded by the Bollywood Luminary and Producer, Jackky Bhagnani, is poised to set the stage ablaze with its inaugural original soundtrack (OST) for the much-anticipated cinematic masterpiece, "Mission Raniganj- The Great Bharat Rescue starring Akshay...
The ultimate celebration of India’s rock music scene: Mahindra Independence Rock unveils its line-up of 10 diverse rock acts for its 29th edition
Following last year’s triumphant return, where Mahindra Independence Rock (I-Rock) reclaimed its rich legacy, India’s legendary rock festival is gearing up to ignite the stage (and mosh pits) once more at Mumbai’s Bayview Lawns this year. This iconic festival’s...
Mark your calendar for the intensive 2-day tattoo workshop and take your skills to the next level!
Join us for an intensive 2-day tattoo workshop and take your skills to the next level! Explore business growth hacks, advanced tattooing techniques, and digital art concepts to enhance your craft. Hosted at Malad, this workshop will provide a unique opportunity to...
Amazon miniTV announces ‘Builders – An Inside story of a modern-day gym!
Amazon miniTV - Amazon’s free video streaming service has been a part of the hit parade with its path-breaking and diverse content library. Adding yet another feather to its hat, the streaming service today announced its latest show, Builders - a unique one-of-a-kind...
Gulf Coast Records’ Blues Music Award-Winner Mike Zito Set to Record New Album Sessions for Upcoming Life Is Hard CD Scheduled for February, 2024
Gulf Coast Records announces a February, 2024, release for the upcoming disc from multi-Blues Music Award-winner Mike Zito, to be titled Life Is Hard. Zito will record the new album September 18-22 during sessions at Sunset Sound Studios in Hollywood, California, with...
Noise announces introduction of Noise Junior, a dedicated smartwatch category for kids in India; launches Noise Explorer
Noise, India's No.1 smartwatch brand, has debuted Noise Junior, the brand's dedicated smartwatch category for kids. Being one of the first to identify the need for smart wearables for kids, Noise today unveiled this new category is tailor-made keeping the children and...
The ultimate celebration of India’s rock music scene: Mahindra Independence Rock unveils its line-up of 10 diverse rock acts for its 29th edition
Following last year's triumphant return, where Mahindra Independence Rock (I-Rock) reclaimed its rich legacy, India's legendary rock festival is gearing up to ignite the stage (and mosh pits) once more at Mumbai's Bayview Lawns this year. This iconic festival's...
DBZ Figure Son Goku Black Hair Version Gift
"DBZ Figure Son Goku Black Hair Version Gift" Celebrates the Legacy of Dragon Ball Z with a Unique Collector's Item Fans of the legendary Dragon Ball Z series have a reason to rejoice as [Your Company Name], a leading name in collectibles, unveils an exquisite new...
Sports
Chotrani, Anahat emerge champions
Maharashtra’s Veer Chotrani and Delhi’s Anahat Singh maintained their imperious form in the tournament, right till the end, lifting the men’s and women’s singles titles in the 6th Cello NSCI Open National Circuit squash tournament, hosted by NSCI at their glass backed...
magicpin Delivers on Its Promise: Office Signage Transformed into ‘Mahipin’ as MS Dhoni and CSK Triumph in IPL Final
Brace yourselves for the epic transformation that just took place at magicpin - India's first and largest hyperlocal startup headquarters in IFFCO Chowk at Gurugram. In a bold move, magicpin’s office signage has officially become 'Mahipin' to honor the incredible...
Chotrani ousts top seed Baitha to make men’s final
Maharashtra’s Veer Chotrani stormed into the men’s final with a dominating upset of top seed Rahul Baitha in the 6th Cello NSCI Open National Circuit squash tournament, hosted by NSCI at their glass backed squash courts, here, on Tuesday. The tournament is...
Sakib gets better of Chauhan to enters semis
Jamal Sakib of Services, seeded 5/8, scored a minor upset over his Services opponent Vaibhav Chauhan (3/4) to enter the men’s semi-finals of the 6th Cello NSCI Open National Circuit squash tournament, hosted by NSCI at their glass backed squash courts, here, on...
Singhva knock out top seed Achpal
Unseeded Naresh Singhva of Maharashtra pulled off the biggest win of his career when he knocked out top seed Adith Achpal of TN in the boys Under-19 pre-quarter-finals of the 6th Cello NSCI Open National Circuit squash tournament, hosted by NSCI at their glass backed...
JioCinema Equals the World Record for Concurrency Clocking 2.5 Cr Viewers During the Qualifier 1 between Gujarat Titans and Chennai Super Kings
JioCinema continued to set new benchmarks as it clocked 2.5 Cr. concurrent viewers during the Qualifier 1 of the TATA IPL 2023 match between Gujarat Titans and Chennai Super Kings. JioCinema not only broke its own record for the third time this season but also...
adidas is unveiling Move For The Planet
Today, adidas is unveiling Move For The Planet; a new global initiative that will harness the collective activity of sporting communities across the world. adidas is encouraging people to turn activity into action as it pledges to donate €1 to Common Goal for every 10...
adidas and Arsenal reveal the Arsenal home jersey
Today, adidas and Arsenal reveal the Arsenal home jersey for the 2023/24 season, marking the 20th anniversary of the ‘Invincibles’ season with a bold new look. Taking design inspiration from the kit worn by the record-breaking team, the jersey features a new shade of...
Reebok launches ‘I am the New’ brand campaign, ropes in leading actor Taapsee Pannu and World’s No. 1 T20 batter Suryakumar Yadav as brand ambassadors
Reebok, a brand synonymous with sports and fitness, has announced a new chapter in its journey. Under the aegis of Aditya Birla Fashion and Retail Limited (ABFRL) in India, Reebok is re-establishing itself as a leading sports and performance brand with a powerful new...
TATA IPL 2023 on JioCinema Clocks a Record-Breaking 1300 Cr. + Video Views in the First Five Weeks
JioCinema, the Official Digital Streaming Partner of TATA IPL 2023, continues to set global benchmarks in the world of digital sports viewing as it clocked over 1300 Cr. video views in the first five weeks. Viewers were glued to JioCinema’s fan-centric presentation as...
Philips Avent encourages mothers to comeback their way with Sania Mirza; launches #AskAvent – a knowledge hub for breastfeeding mothers
Motherhood is one of the most sacred and revered roles that a woman can take on and the journey is even more incredible. However, it comes with its unique set of challenges that can often make it difficult for mothers to pursue their passions and careers. After having...
Pradhan, Gawate win Federal Bank Kochi Marathon 2023
Uttarakhand's Arjun Pradhan and Maharashtra long-distance runner Jyoti Gawate won the inaugural Federal Bank Kochi Marathon 2023, which was organized by CleoSportz, and held here on Monday. Arjun Pradhan, who was the favourite in the men's elite category, emerged...
This 19 years old Three-Star Wide Receiver Deserves Your Attention
There are always players that don’t end up getting the attention they rightfully deserve in both college and professional sports. Here we are hoping to change that regarding one player in particular, DeVante Perkins. Perkins is a name that, if you aren’t already...
National Lacrosse League Announces Information For 2022 Entry Draft
The National Lacrosse League (@NLL), the largest and most successful professional lacrosse property in the world, today announced that the 2022 NLL Entry Draft will be held on Saturday, September 10 at 2 p.m. ET at the historic The Carlu in downtown Toronto....
Joe Moore Award Becomes First Post-Season College Award Platform To Launch NIL Program
The Foundation for Teamwork, owners and creators of the Joe Moore Award (@joemooreaward) which honors the most Outstanding Offensive Line Unit in College Football, today announced that it is believed to be the first post season college award program to launch a Name...
Interviews & Features
Blackjack Review 2022 For Online Gambling- Gambling Sites Club
You’ve probably heard that learning blackjack takes a minute and mastering it takes a lifetime. It’s only fair, because blackjack online is a fantastic game! Right? Let’s take a closer look at this contentious game. What is Online Blackjack Gambling? Blackjack is a...
3 Reasons Why It is a Must to Hire an eCommerce Design Agency
While Considering the website composition and manner in which a web-based store looks, typically the creative viewpoints ring a bell. How a specific picture looks or the varieties that are being utilized. These are absolutely significant components, however there are...
Year Ender Quotes | Women Entrepreneurs
Malini Agarwal- Founder & Creative Director MissMalini Entertainment & Girl Tribe From the onset of the pandemic there has been a spotlight on influencer marketing as a crucial part of the marketing mix. The rapid growth of the creator ecosystem over the last...
‘Puressentiel Purifying Air Spray’ an essential requirement for your home
Pollution has been a concern for us Indians more so over the past few years with the AQI rising above hazardous. It is well known that outdoor polluted air can cause irreversible damage to the respiratory system. However Indoor air pollution is 100 times more...
Recipe – CORNITOS AVOCADO MINI WAFFLES WITH DRAGON FRUIT AND AVOCADO SALSA
Avocado Mini Waffles: Ingredients: For the avocado filling: 1/2 mashed avocado 1/4 cup grated paneer 1 tbsp chopped onion 2 chopped garlic cloves 1/2 chopped green chilli 1/2 tsp mixed herbs 1 tbsp chopped coriander 1/2 tsp lemon juice Salt to taste For waffles:...
Card91 appoints Srijit Sanyal as Vice President, Sales
Card91, a payment infrastructure platform, has appointed Srijit Sanyal as Vice President, Sales & Partnership. A veteran in the Fintech industry, Srijit has nearly 2 decades of rich sales experience with banks, fintechs and pharmaceuticals and has held senior...
All News
Rihanna and PUMA Get Down to Earth with the Creeper Phatty Earth Tone
The FENTY x PUMA Creeper Phatty Earth Tone has arrived with luxe, nubuck materials and touches of...
Crown Digital Spearheads the Future of AI in F&B with Ella the Robobarista at AIM Global
Crown Digital, Singapore’s first full-stacked F&B tech startup and the creator of Ella the Robot...
Microex Launches Web3.0 Financial Trading Solution – Pioneering Innovation in Financial Technology
NEW YORK, Apr 17, 2024 - (ACN Newswire) - Microex, a UAE-based financial technology company, today...
The Executive Centre Announces Record Revenue in FY2023 Annual Results
The Executive Centre (TEC), the leading premium workspace provider that serves more than 47,000...
All-New Triton Confirmed as First Double-Cab Pickup Truck to Achieve 2024 Five-Star ANCAP Safety Rating
Mitsubishi Motors Corporation (hereafter, Mitsubishi Motors) announced that the all-new Triton(1)...

Kalimat Telecom Selects Parallel Wireless for Network Build Out
New Delhi, Delhi, India Parallel Wireless, Inc., the leading provider of the world’s first...



Parallel Wireless’s All-G Technology Ready to Transform Telecom Services in India
New Delhi, Delhi, India Parallel Wireless, Inc., the leader in providing the world’s first...



Quickparts Achieves Global ISO 27001:2022 Certification, Highlighting Commitment to Information Security
The ISO 27001:2022 certification signifies that Quickparts has implemented a robust Information...



Unveiling Sapphire, The First True Native Edge Ai Camera Solution, By Kami Vision: A Glimpse Into The Future Of Security
Launching SAPPHIRE! Delve into the cutting-edge of intelligent business technology at ISC WEST...



R-mb-rger Family Association Lauds Grit in Facing Challenges at 2024 Reunion
Local historian and author Steve Troutman and Family Historian Bob Averell will be joined by guest...



Philadelphia Phillies and NEST launch Skilled Trades All-Star Program to Ignite Interest in Skilled Trades Among Youth
Local Students to Gain Hands-On Experience at Citizens Bank Park on April 24 and August 1 The...



Dr. Phyllis Worthy Dawkins, HBCU Executive Leadership Institute Executive Director, To Receive the Johnson C. Smith 2024 ‘Arch of Triumph’ Award
Clark Atlanta University’s HBCU Executive Leadership Institute (HBCU ELI) proudly announced that...



Melville Law Represents Syndicate of Venture Capital Funds in $6M Seed Investment for Brain Jar Games
$6 million investment from venture capital funds syndicate targeted on boosting the innovative...



One Way Exteriors Announces Relocation to a New Office in Wilkesboro, NC
One Way Exteriors, a leading seamless gutter company serving the North Carolina area, is excited...



Bemis Assist Premium Toilet Seat Eliminates Need for Wall-Mounted Rails
Designed for safety and independence in the bathroom, Assist features secure, built-in support...
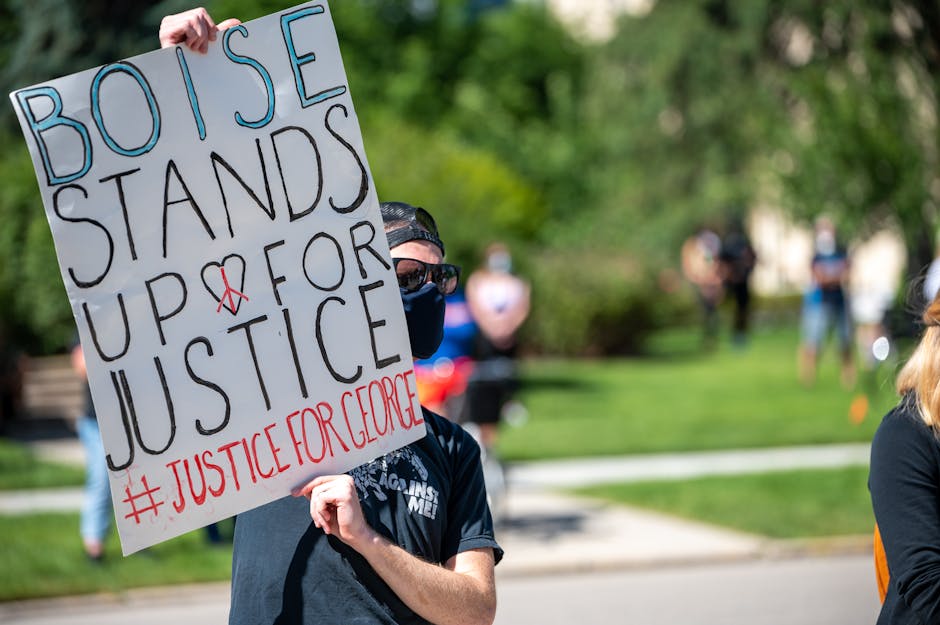
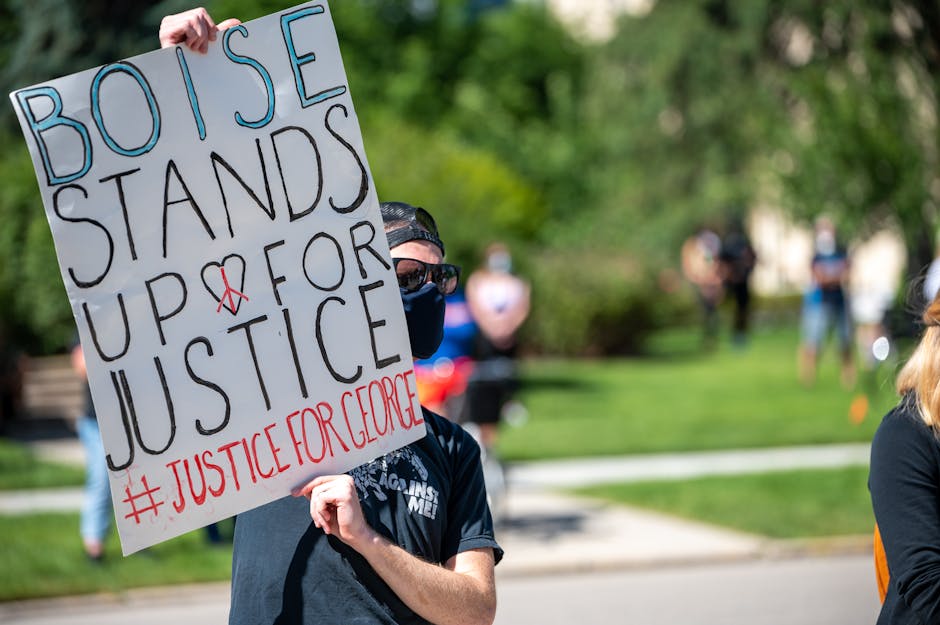
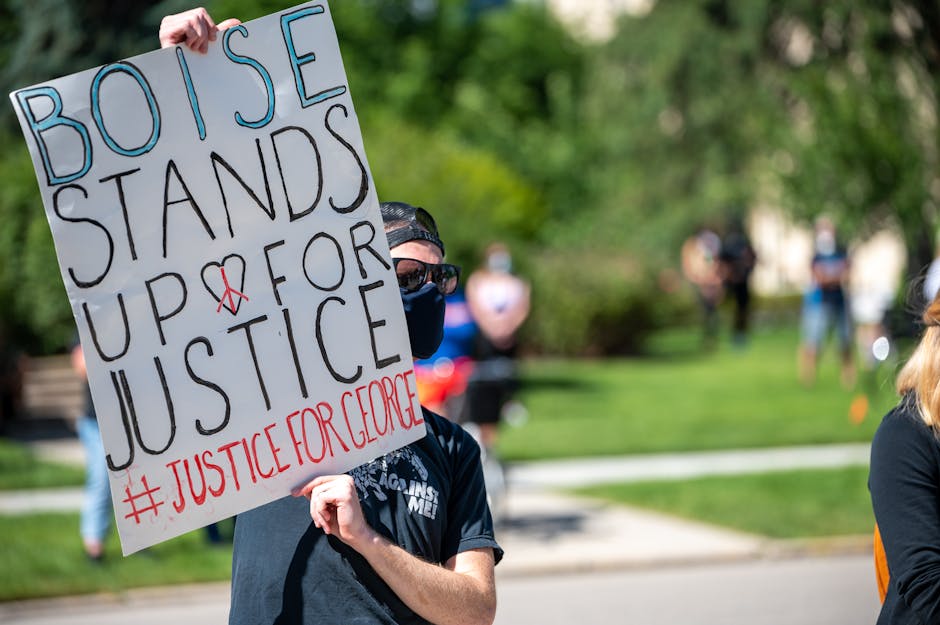
L-Tron to Attend and Sponsor SC IAI Investigators Conference
FORT MILL, S.C. - April 16, 2024 - PRLog -- For the second consecutive year, L-Tron will attend...

100K-SF Office Complex in Las Vegas to be Converted Into Food-Driven Lifestyle Center
The Cliff Outdoor Dining LoungeLAS VEGAS - April 16, 2024 - PRLog -- Partners Capital and CNR...


Ocean Partnership for Children Celebrates Autism Awareness and Acceptance Month
Autism Awareness MonthTOMS RIVER, N.J. - April 16, 2024 - PRLog -- As April unfolds, so does a...
Adani Family Completes Ambuja Warrant Subscription Infuses INR 20,000 Cr to increase stake to 70.3%
Ambuja Cements Ltd (Ambuja), the cement and building materials flagship company under Adani...
South Indian Bank signs MOU with Ashok Leyland Limited for Dealer Financing
South Indian Bank has signed an MoU (Memorandum of Understanding) with Ashok Leyland Limited for...
Suzuki Motorcycle India expands its footprint in Kerala
Further expanding its reach in Kerala, Suzuki Motorcycle India Pvt. Ltd. (SMIPL), the two-wheeler...

A4 Sportswear Makes Massive Move to Sustainable Apparel
A4, manufacturer of premium sportswear and team uniforms, is proud to announce that it is now...


DSI’s Urban Operations Summit Announced
The 2024 Urban Operations Summit will focus on the tools, techniques, and training required to...


the*gamehers Launches an Ambassador Program in Conjunction with Groundbreaking Partnerships
the*gamehers continues to make a positive impact in the gaming community with its newly developed...


Marine Silver Star Recipient Honored by Military History Institute
Today the city of Escondido will unveil a bronze bust of former resident and hometown hero...
Contribute to Web Newswire
Access Premium Content
You can access and use the content for free on your website if you give an attribution and linkback to us.
Thanks for supporting us!
Contribute your Content
PR Agencies, Brands and others can contribute your content with us for free. This will now be subject to editorial approval. (5pm-6pm IST, All Days in a Week)
Follow Us
Please link us using RSS. We have stopped updating social medias channels for updates so that we can focus on quality content that is more useful for all of us.
